Health Canada approves Ottawa company’s rapid coronavirus test kits
Posted April 13, 2020 6:19 am.
Last Updated April 14, 2020 12:31 pm.
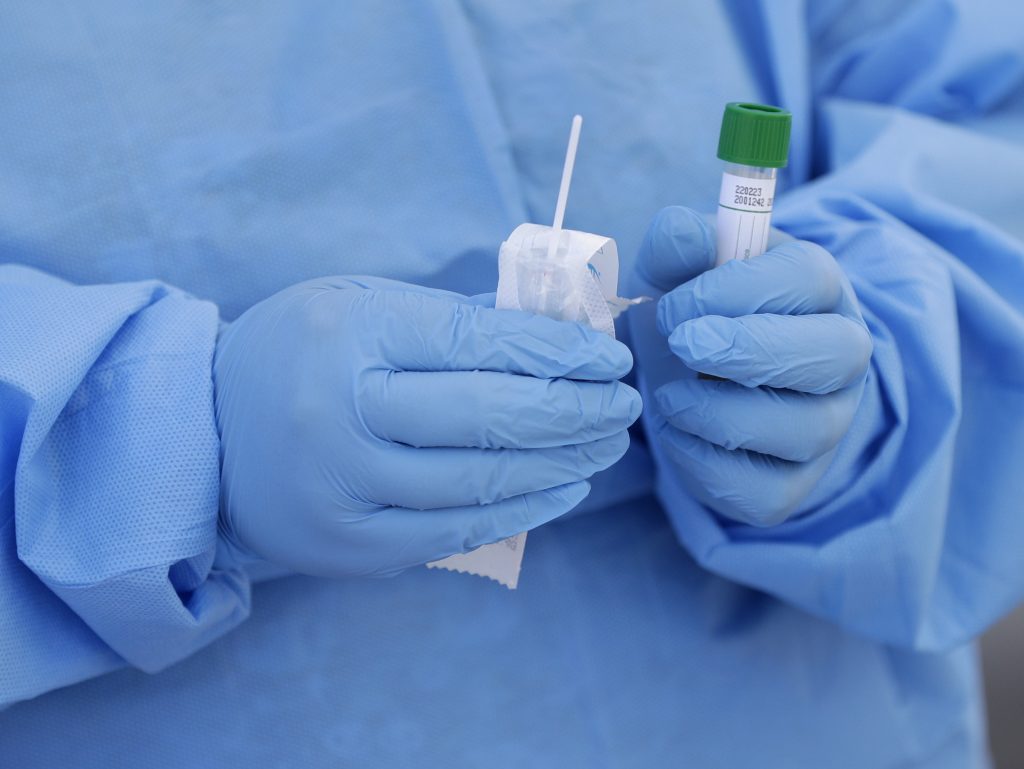
A rapid, portable testing device for COVID-19 developed by an Ottawa company has received approval from Health Canada.
The device, which was developed by Spartan Bioscience, is a handheld DNA analyzer that allows hospitals and other institutions to independently test patients and receive results without having to send the samples away to a provincial or national lab.
The device comes with its own test cartridges and proprietary swabs, which are manufactured in Ottawa.
The test can be administered by “non-laboratory personnel” in places such as airports, border crossings, doctors’ offices, pharmacies, clinics, and remote communities.
In a release, the company said the tests can now be shipped to “Spartan’s federal and provincial government partners starting immediately.”
“We are grateful to the Government of Canada for working closely with us to expedite the review and approval process,” Paul Lem, CEO of Spartan Bioscience, said in a release.
“We are ready to start shipping our portable COVID-19 test to the federal and provincial governments, and to make them widely available to Canadians.”
A worldwide shortage of medical swabs has slowed down the traditional testing, which is especially being felt in Ontario. Until recently, the province has had the lowest testing rate for COVID-19 in Canada.
RELATED: Coronavirus and health
- Coronavirus FAQ: what it is, how it spreads and who to call
- How to self-isolate amid the COVID-19 outbreak
- ‘Cruel times’: How coronavirus has changed the way we live, die and grieve
- Tips to protect yourself from COVID-19
- Experts say ‘immune-boosting’ claims won’t help ward off coronavirus
- Map of Ontario’s coronavirus assessment centres










