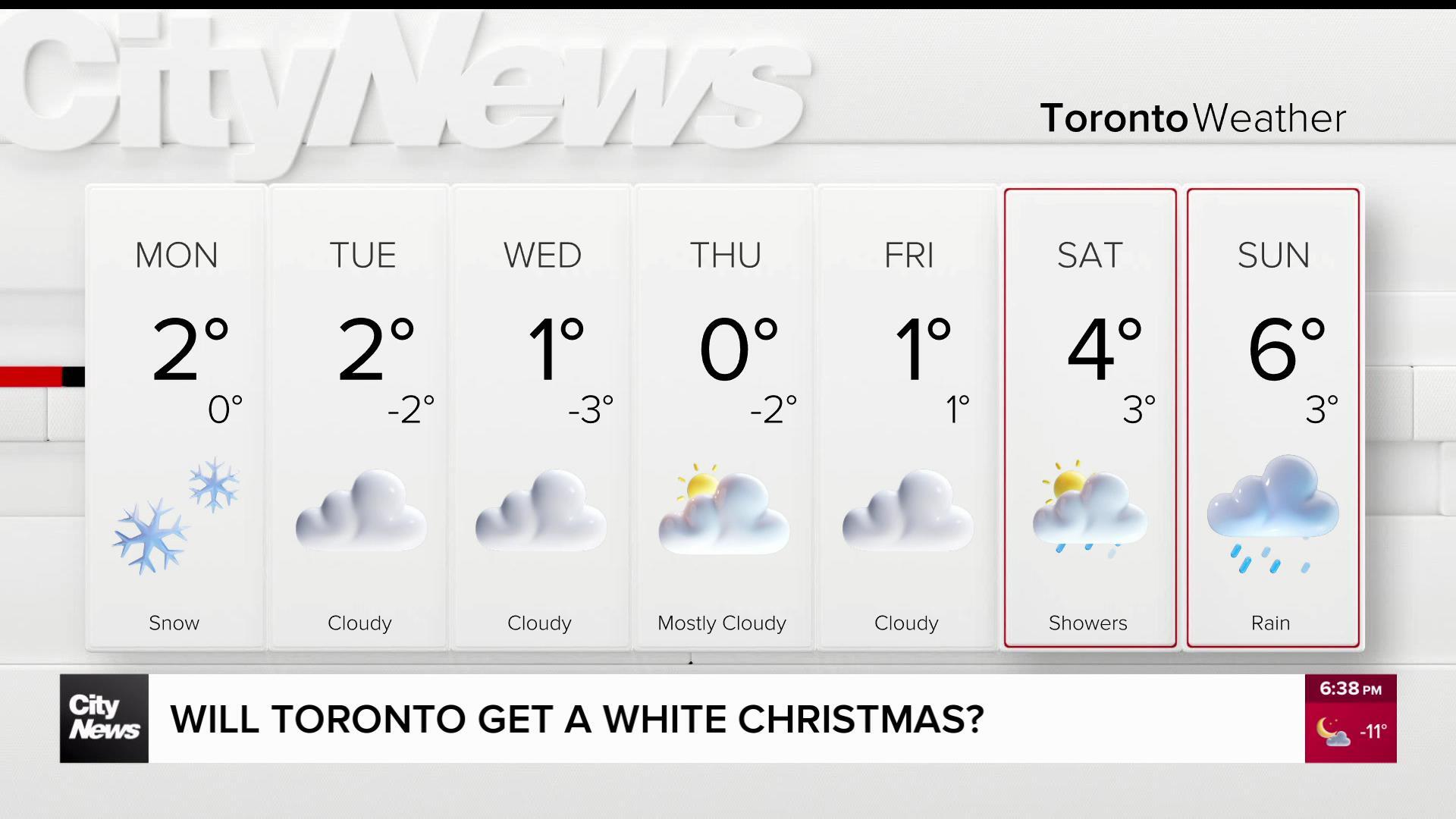
Health Canada approves rapid test for COVID-19

Posted September 30, 2020 1:52 pm.
Last Updated September 30, 2020 4:24 pm.
Health Canada has given the green light to a rapid test for COVID-19.
The department posted news of the approval of the Abbott Diagnostics ID Now test this afternoon, a day after the government said it had a deal to buy nearly eight million of the tests from the company.
The deal was conditional on Health Canada’s approving the tests, which it has done today.
The Trudeau government did not give a timeline for when these test kits would be delivered to the provinces and territories. Yesterday, Canada's Chief Public Health Officer Dr. Theresa Tam did say remote and high risk settings would have priority #cdnpoli #COVID19
— Cormac Mac Sweeney (@cmaconthehill) September 30, 2020
The test has been in use in the United States for several months already.
Abbott’s website says the test can produce results in less than 13 minutes in the same place a nasal swab is taken from a patient.
Ontario Premier Doug Ford, who has been calling for quicker approval from the federal government on rapid testing, called the announcement a “great first step” when it comes to expanded testing options in Canada.
“What we need now is more information about when we can expect the federal government to deliver these units where they’re needed most, including remote and indigenous communities, long-term care homes and other congregate care settings at highest risk of experiencing outbreaks,” Ford said in a statement.
“We don’t have a moment to spare as cases continue to rise.”
Ford also called on the federal government to approval additional rapid test kits such as the BinaxNOW antigen test from Abbott, which has already been approved for use in the United States. That test is described as a highly portable credit card sized test kit that provides results in 15 minutes.
In Question Period, Prime Minister Justin Trudeau said the approved tests would be distributed to provinces and territories “in the coming weeks.”
Health Canada has emergency authority to quickly approve tests for COVID-19 and has been under increasing pressure to allow the use of rapid testing in Canada as cases surge and Canadians are sometimes waiting days to get their test results.