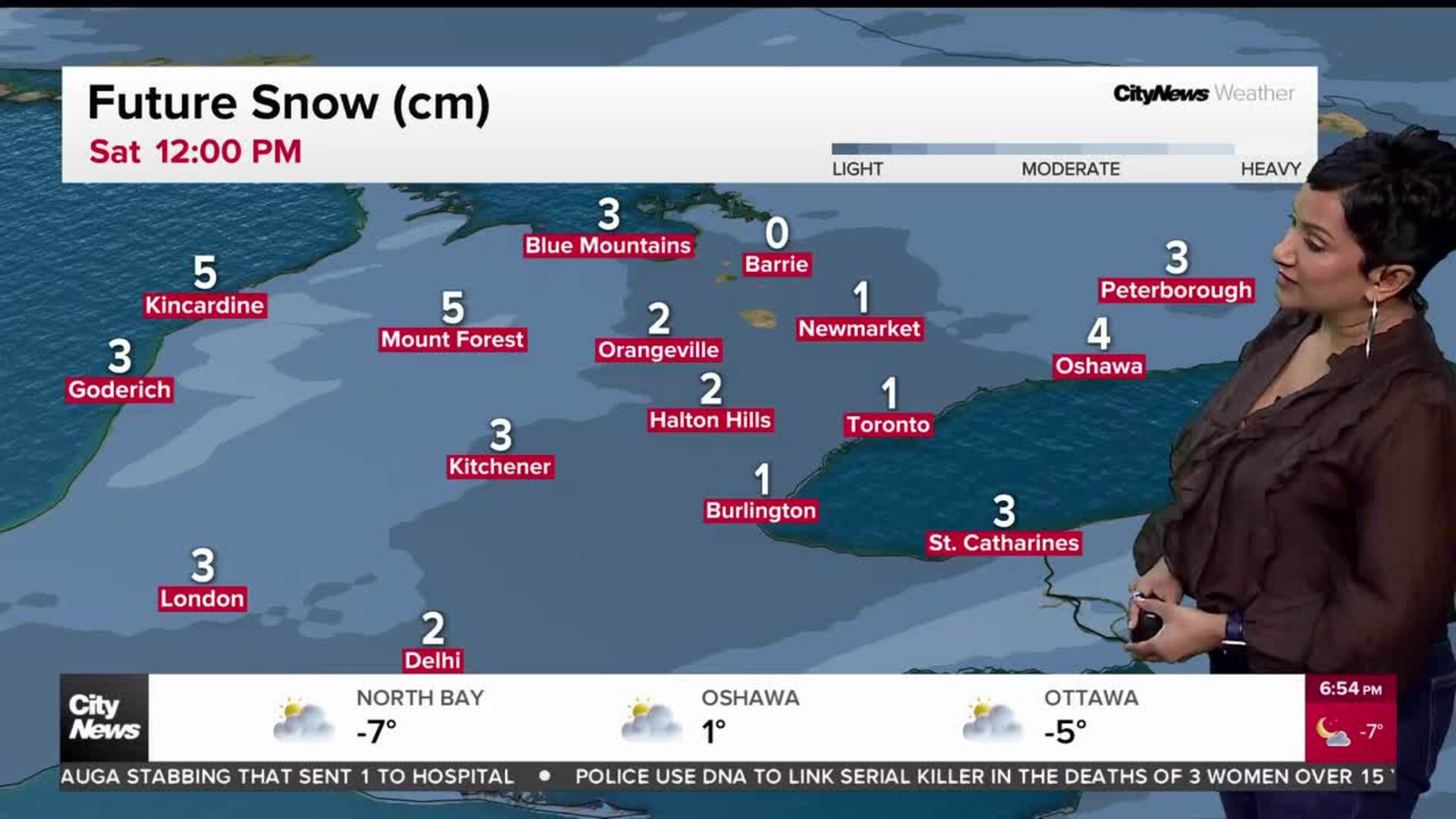
NACI recommends halting AstraZeneca COVID-19 vaccine for those under 55

Posted March 29, 2021 11:55 am.
Last Updated March 29, 2021 5:07 pm.
Canada’s National Advisory Committee on Immunization (NACI) recommends suspending the use of the Oxford-AstraZeneca COVID-19 vaccine for anyone under the age of 55 as a precautionary measure, pending further investigation.
The recommendation comes after more reports that patients in Europe developed blood clots following vaccination.
Cases were identified primarily in women under the age of 55, but cases in men have also been reported and were typically seen between four and 16 days after receiving the vaccine.
Health Canada says it has not received any reports of blood clots in Canada to date.
However, the agency says it now requires that the manufacturers of the AstraZeneca and Verity pharmaceuticals Serum Institute of India vaccine conduct a detailed assessment of the benefits and risks of the vaccine by age and sex in the Canadian context.
“This info will support the ongoing evaluation of these blood clotting events and allow Health Canada to determine if there are specific groups of people who maybe at higher risk,” said the agency’s chief medical adviser Dr. Supriya Sharma.
She added that the regulators are not pulling AstraZeneca’s approval in Canada, but additional terms and conditions may be forthcoming based on the results of the study.
RELATED: EU regulator reviews AstraZeneca shot and blood clot links
The incidence of blood clots following vaccination is called vaccine induced prothrombotic immune thrombocytopenia (VIPIT).
“The exact mechanism by which the AstraZeneca vaccine triggers VIPIT is still under investigation and no other risk factors have been consistently identified in patients who developed VIPIT,” says Dr. Shelley Deeks from NACI. “This adverse event has not been identified following either MRNA COVID vaccine to date.”
Dr. Deeks said the rate of the adverse events is unknown at this time but based on information from Europe, it was originally estimated to occur at approximately one per million people vaccinated with AstraZeneca. However a higher rate of one per 100,000 has been reported by the Paul Ehrlich Institute in Germany.
“Following a population-based analysis, considering what we know of VIPIT, assessing the risk of COVID-19 disease in Canada by age and considering that alternate vaccines are available in Canada, NACI has determined that there is substantial uncertainty about the benefit of providing AstraZeneca COVID-19 vaccine to adults under 55 years of age given the potential risks associated with VIPIT,” said Dr. Deeks.
Adults 55 and older can still be offered the vaccine with informed consent due to the increased risk of severe cases of COVID-19 in this age group and as VIPIT appears to be rarer.
She added that case fatality for VIPIT is approximately 40 per cent but may decrease with increased awareness of VIPIT and early treatment.
Earlier Monday, Health Minister Patty Hajdu told a videoconference that Canada is closely monitoring investigations into possible adverse effects linked to the vaccine that are taking place in several jurisdictions.
Prince Edward Island suspended its use of the shot for those aged 18 to 29 earlier in the day. The province’s health officials said in a brief statement the vaccination appointments are on hold pending the updated information from Health Canada and the vaccine committee.
Quebec also announced plans to stop using the AstraZeneca vaccine shortly after.
Ontario Premier Doug Ford said his government will follow the lead of the federal government.
“If it’s going to put anyone at harm, we just won’t use it,” he said.
Ford added that he “would rather wait,” if it means a few months, for Pfizer, Moderna and Johnson & Johnson than “roll the dice” with AstraZeneca.
No one below 60 years of age has received the AstraZeneca vaccine in Ontario.
Ontario’s Chief Medical Officer said he and many others had been working on the situation all weekend and called it “very rare and unique.”
“We have said that we are pausing on the use at this time for those under 55 because while there is a very rare incident, we want to make sure we’re fully appraised on this,” he said. “Because if we’re going to give people advice, we’d like to give them the best advise we can give them on this matter so they are as informed as we are.”
In Toronto’s daily COVID-19 briefing, the Medical Officer of Health said she was very interested in the specifics of the recommendation from Health Canada and NACI and added that the revising of guidelines is a good thing.
“It’s important that we have this activity happening, that there is a National Advisory Committee on Immunization, constantly assessing and reassessing vaccines,” she said. “We expect that our federal partners who are responsible for the regulation of vaccine use in Canada continue to play that very important role and I am certainly assured, as should the people of Toronto and beyond, that there is active and constant assessment of the safety and effectiveness of vaccines.”
The news is the latest setback for a vaccine that has been plagued by questions over its preliminary trial data in the United States, confusion over whether it is safe for seniors and reports that it is linked to blood clots in a small number of people.
RELATED: AstraZeneca COVID-19 vaccine recommended for those aged 65 and older: NACI
It comes as Canada is expected to receive 1.5 million doses of the vaccine from the United States on Tuesday.
Canadian health officials first recommended the AstraZeneca vaccine for those under 65, but on March 16 they adjusted their advice to say that it could also be given to seniors.
Several European countries suspended the use of the vaccine as a precautionary measure earlier this month amid concern that its use could be linked to blood clots in a few patients, although many countries have resumed its use after the European Medicines Agency said it was safe.
Health Canada updated its product label for AstraZeneca to warn about blood clotting last week but said reports of those events were nonexistent in Canada and very rare elsewhere.
The label warning followed reports from Europe that the vaccine might cause a rare type of blood clot in the brain in a very small number of patients.
“Health Canada’s guidance issued to health-care practitioners last week still stands, and provides vaccine recipients with information on the signs and symptoms to monitor for following vaccination,” said Dr. Sharma.