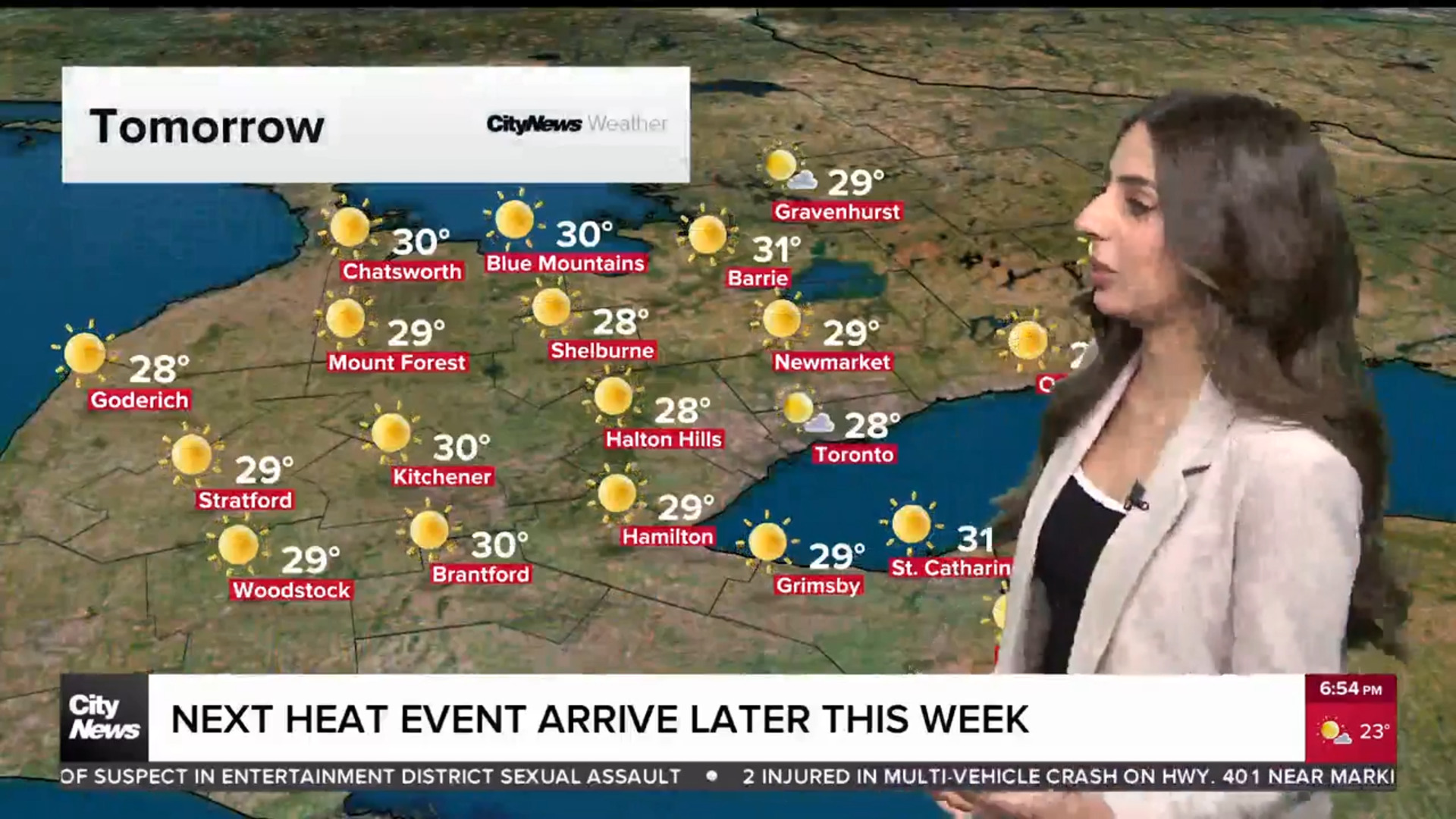
New gene therapy being developed at University of Toronto could treat MS
Posted May 3, 2023 9:43 pm.
Canada has one of the highest rates of Multiple Sclerosis (MS) in the world — a chronic condition for which there is no cure. But a new research breakthrough at the University of Toronto has the potential to improve the lives of those living with the disease far beyond current medications.
A research team led by neuroscientist Maryam Faiz has developed a “reprogramming technology” that converts cells involved in the progression of MS in the central nervous system (CNS) into cells that promote functional recovery.
Faiz explains that there are three main types of cells in the CNS: astrocytes, oligodendrocytes and neurons.
“Astrocytes and oligodendrocytes are part of a class of cells that can make more of themselves in the brain, and neurons are static,” she says.
Oligodendrocytes produce myelin — the insulating coating that wraps around nerve cells and is really important for fast neuronal transmission or fast signalling within the brain. In some cases, some astrocytes become “neurotoxic,” attacking and degrading the myelin.
“Then you can get associated loss of function depending on the location. So you can have difficulty speaking, talking, running, or walking,” says Faiz.
“So our research is focused on developing a type of gene therapy, and the hope is that it can reverse some of the functional impairments seen in MS patients.”

Neuroscientist Maryam Faiz observes the results of an experiment involving a new gene therapy that could have applications in the treatment of MS. CITYNEWS/Dilshad Burman
Simply put, Faiz says they’ve discovered a way to turn “bad cells” into “good cells.”
“Our body is comprised of different cell types, and so each one of those different cells is instructed on who to be by a set of instructions or a set of codes. And so the premise of reprogramming is really simple. If you know those instructions or, you know, those codes, you can basically tell any cell to become what you want it to be.”
She explains that different cells can perform various functions at different times. The hope is to identify cells that are causing harm at a specific stage of the disease and turn them into cells that are beneficial at that same stage instead — thereby making the therapy highly customizable.
“In theory, you could fine-tune it to whatever you needed. And so if you think of it like a bespoke therapy or a personalized approach to repair, I think that’s actually the most exciting part,” she says.
“If we can pinpoint those cells that need to be removed and the cells that need to be created, we can design therapeutics that are specific to not only broadly MS but to every stage of MS, different types of MS and then for other diseases like Alzheimer’s or spinal cord injury.”
Commonly, oligodendrocyte cells are grown in a dish, which are then transplanted into the body. The patient needs to be prescribed immunosuppressive drugs to survive in the body.
“But what’s amazing about this technology is that you can actually do that at the site of disease in the brain or the spinal cord or even the optic nerves … we’re doing this two birds with one stone approach to brain repair. We’re removing the harmful cells and putting back the beneficial cells [simultaneously],” says Faiz.

Justine Bajohr conducts an experiment involving a gene therapy that could have applications in the treatment of MS. CITYNEWS/Dilshad Burman
If all goes well, the therapy may be five to ten years away from clinical trials.
“We think that it could be curative. There’s still more science to be done, [but] the hope is that eventually, it could turn into a cure — at least slow down the progression of MS without using daily drugs,” she says.
Mark Gonzalez, who was diagnosed with MS at the age of 27 last September, says it gives him hope for the future.
“I am undergoing treatment with Ocrevus, which is an immunosuppressive drug … and of course, there are tons of side effects that come along with that. I do have to be extremely cautious, especially in the world that we’re in now. Even viruses like COVID-19 that are still around — now that I am immunocompromised — can affect me significantly. So finding a solution where maybe something like that doesn’t have to be the only option is very exciting,” he says.
Immunologist Shannon Dunn, a collaborator on the project with Faiz, echoes those sentiments.
“My mom was diagnosed with MS in the early 1970s. Back then, there was nothing for MS patients. So the neurologist would have to deliver the bad news, and it never got better,” she says.
“It’s really exciting to see new technologies come forward, and people like Maryam outside the field come in and try new things. That’s essentially how discoveries are made.”
Dunn is hopeful that if the therapy works, it will be a valuable addition to the toolbox for battling MS.
“It’s an autoimmune disease, so there’s still going to be autoimmune attacks. We already have stuff that deals with that,” she says.
“But this could be potentially an add-on to help patients repair damage after they’ve had these autoimmune attacks so that 10 or 20 years down the road, they don’t enter the progressive phase of the disease, that they live longer in a healthy state, into natural aging.”
Gonzalez adds that knowing that the research into therapies that could have potential applications to MS is moving in the right direction encourages him to stay positive.
“We can’t dwell on the fact that this is something we have to deal with,” he says.
“We have to also look forward to the research being done and be supportive of those things, donate to those causes and do as much as we can to ensure that that continues to progress so that we can change lives down the road.”